Title
How is the capacity of roots to extract water from the soil?
Who
Andrea Carminati Chair of Soil Physics, University of Bayreuth, Germany
Introduction
How easily do plant roots extract water from the soil? When does the soil becoming a limiting factor for root water uptake? What root and rhizosphere traits favour water uptake? A key variable to answer these questions and understand soil-plant water relations is the leaf water potential. But, non-destructive measurements of leaf water potential are challenging. We need to measure the difference in water potential between soil and plants.
Research question: Do root hairs help roots take up water from the soil? Despite the well-documented role of root hairs in phosphate uptake, their role in water extraction is controversial (Passioura 1980).
 Fig. 1 Plant pressure ecotron (Umwelt-Geräte-Technik GmbH, Germany)
Fig. 1 Plant pressure ecotron (Umwelt-Geräte-Technik GmbH, Germany)
 Fig. 2 Hairless mutant (left side) and wild type (right side)
Fig. 2 Hairless mutant (left side) and wild type (right side)
Material and Methods
The Plant-Pressure-Ecotron (PPE) allows accurate measurements of leaf water potential and transpiration of intact plants exposed to varying evaporative demand and soil drying (Fig. 1). PPE measures the balancing pressure that is needed to bring the leaf xylem to atmospheric pressure, which is equivalent to the leaf xylem suction prior to pressurization. PPE allows to measure the hydraulic conductance of the soil-plant system and its components, making the PPE an optimal method to study plant tolerance to drought conditions.
Barley (Hordeum vulgare cv Pallas) and its root-hairless mutant brb were grew in a plant pressure chamber (Fig. 2), whereby the transpiration rate could be varied whilst monitoring the suction in the xylem. A pot was placed into the pressure chamber. The plant shoot, which remained outside the pressure chamber, was enclosed in a cuvette (Fig. 3) that had an internal fan to create mildly turbulent air flow at 23°C. The plant was illuminated horizontally by a light-emitting diode (LED) lamp. The transpiration rates were determined by multiplying the flow rate through the cuvette by the difference in humidity between the ingoing and outgoing air. Sufficient pneumatic pressure (the ‘balancing pressure’ P) was applied in the root chamber to bring the exposed xylem in a trimmed leaf to the point of bleeding. Plants were kept at balancing pressure as the soil dried by an automatic electronic controller. A sensor attached to the trimmed leaf transmitted a signal to the controller which regulated the chamber pressure to maintain the xylem sap at the point of bleeding, with a precision of c. 5 kPa and a range from 0 to 2400 kPa.
The method provides accurate measurements of the dynamic relationship between the transpiration rate and xylem suction (Carminati, Passioura et al. 2017).
 Fig. 3 Schematic of equipment used to measure pressure drop across the plant and soil. The infrared emitter and detector form a position sensor that monitors the position of the meniscus inside the capillary tube. The position sensor is connected to a pressure controller that regulates the pressure inside the pressure chamber. Drawing not to scale.
Fig. 3 Schematic of equipment used to measure pressure drop across the plant and soil. The infrared emitter and detector form a position sensor that monitors the position of the meniscus inside the capillary tube. The position sensor is connected to a pressure controller that regulates the pressure inside the pressure chamber. Drawing not to scale.
Results and Conclusion
The relationship between the transpiration rate and xylem suction was linear in wet soils and did not differ between genotypes. When the soil dried, the xylem suction increased rapidly and non-linearly at high transpiration rates. This response was much greater with the brb mutant, implying a reduced capacity to take up water (Fig. 4).
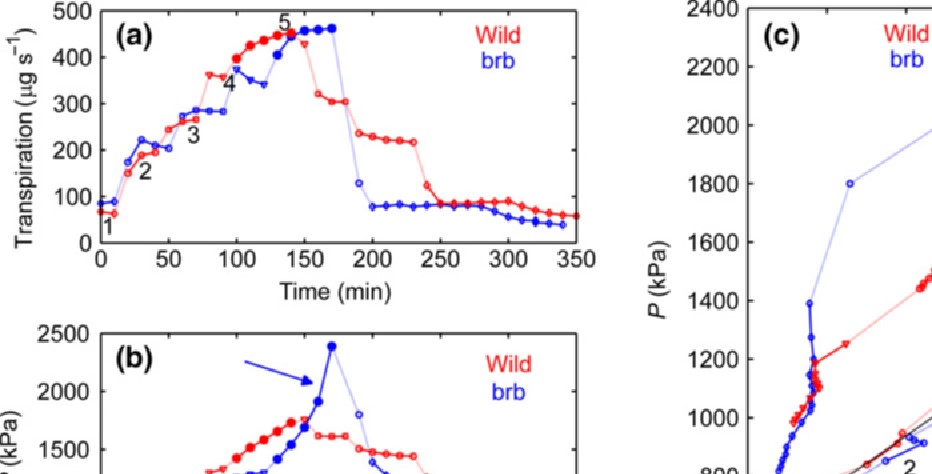 Fig. 4 Comparison of a root-hairless barley brb (Hordeum vulgare) mutant and its wild-type plant. The data shown were collected from a brb and a wild-type plant grown in the sandy loam potting mix at an initial gravimetric water content of hg = 0.17. (a) One cycle of increasing and decreasing transpiration when the plants were placed in the root pressure chamber. Transpiration was modified by varying the humidity and light intensity.
Fig. 4 Comparison of a root-hairless barley brb (Hordeum vulgare) mutant and its wild-type plant. The data shown were collected from a brb and a wild-type plant grown in the sandy loam potting mix at an initial gravimetric water content of hg = 0.17. (a) One cycle of increasing and decreasing transpiration when the plants were placed in the root pressure chamber. Transpiration was modified by varying the humidity and light intensity.(b) Balancing pressure P needed to maintain the leaf xylem at atmospheric pressure. The balancing pressure is numerically equal to the suction that the xylem would have had if the plant had not been pressurized. The time is identical to that in plot (a). The blue
arrow indicates the large increase in P in brb under high transpiration demand. (c) Relationship between P and E. At low transpiration stages (1–4), the P(E) curves for the two genotypes were similar and linear. At stage 5, there was a rapid increase in P in brb (blue arrow), compared with the moderate increase in the wild-type. (please see Table 1 and Fig. 3 inCarminati, Passioura et al. 2017).
Discussion and conclusion
The authors showed that root hairs facilitate the uptake of water by substantially reducing the drop in matric potential at the interface between root and soil in rapidly transpiring plants. The experiments also reinforce earlier observations that there is a marked hysteresis in the suction in the xylem when the transpiration rate is rising compared with when it is falling, and possible reasons for this behavior are discussed in (Carminati, Passioura et al. 2017). Conclusion: Complex biophysical processes at the root-soil interface affect the availability of water to plant roots: Mucilage and root hairs are key elements for the hydraulic connection between roots and soil.
References
Allen CD, DD Breshears, NG McDowell (2015) On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6:1–55. doi: 10.1890/ES15-00203.1
Allen CD, AK Macalady, H Chenchouni, D Bachelet, N McDowell, M Vennetier, T Kitzberger, A Rigling, DD Breshears, EH Hogg, P Gonzalez, R Fensham, Z Zhang, J Castro, N Demidova, J-H Lim, G Allard, SW Running, A Semerci, N Cobb (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660-684. doi: papers3://publication/doi/10.1016/j.foreco.2009.09.001
Breda N, R Huc, A Granier, E Dreyer (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann For Sci 63:625-644. doi: 10.1051/forest:2006042
Carminati A, JB Passioura, M Zarebanadkouki, MA Ahmed, PR Ryan, M Watt, E Delhaize (2017) Root hairs enable high transpiration rates in drying soils. New Phytol 216:771-781
El-Madany T, M Migliavacca, O Perez-Priego, O Kolle, N Carvallhais, Y-P Luo, A Carrara, G Moreno, J Pacheco-Labrador, M-P Martin-Isabel, M Reichstein (2016) MaNiP-A large scale nutrition manipulation experiment in a tree grass ecosystem to understand ecosystem-physiological response to chnanging N/P stoichiometry and water availability. 2nd ICOS Scientific Conference on greenhouse gases and biogeochemical cycles , Helsinki
Ellenberg H, C Leuschner (2010) Vegetation Mitteleuropas mit den Alpen: in ökologischer, dynamischer und historischer Sicht, Utb
Geßler A, C Keitel, J Kreuzwieser, R Matyssek, W Seiler, H Rennenberg (2007) Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 21:1-11. doi: 10.1007/s00468-006-0107-x
Goisser M, U Geppert, T Rötzer, A Paya, A Huber, R Kerner, T Bauerle, H Pretzsch, K Pritsch, KH Häberle, R Matyssek, TEE Grams (2016) Does belowground interaction with Fagus sylvatica increase drought susceptibility of photosynthesis and stem growth in Picea abies? For Ecol Manag 375:268-278. doi: https://doi.org/10.1016/j.foreco.2016.05.032
Hill M, N Hanan, W Hoffmann, R Scholes, S Prince, J Ferwerda, R Lucas, I Baker, A Arneth, S Higgins (2011) Remote sensing and modeling of savannas: The state of the dis-union. Proceedings of the 34th International Symposium on Remote Sensing of the Environment (ISRSE), Sydney, NSW, Australia, pp. 10-15
IPCC (2012) Summary for Policymakers: Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. In: Field CB, Barros V, Stocker TF, Qin D, Dokken DJ, Ebi KL, Mastrandrea MD, Mach KJ, Plattner G-K, Allen SK, Tignor M, Midgley PM (eds) A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK and New York, USA, pp. 1-19
McDowell N, WT Pockman, CD Allen, DD Breshears, N Cobb, T Kolb, J Plaut, J Sperry, A West, DG Williams, EA Yepez (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719-739. doi: 10.1111/j.1469-8137.2008.02436.x
Metz J, P Annighöfer, P Schall, J Zimmermann, T Kahl, ED Schulze, C Ammer (2016) Site‐adapted admixed tree species reduce drought susceptibility of mature European beech. Global Change Biol 22:903-920. doi: doi:10.1111/gcb.13113
Passioura J (1980) The transport of water from soil to shoot in wheat seedlings. J Exp Bot 31:333-345
Perez-Priego O, TS El-Madany, M Migliavacca, AS Kowalski, M Jung, A Carrara, O Kolle, MP Martín, J Pacheco-Labrador, G Moreno, M Reichstein (2017) Evaluation of eddy covariance latent heat fluxes with independent lysimeter and sapflow estimates in a Mediterranean savannah ecosystem. Agric For Meteorol 236:87-99. doi: https://doi.org/10.1016/j.agrformet.2017.01.009
Poulter B, D Frank, P Ciais, RB Myneni, N Andela, J Bi, G Broquet, JG Canadell, F Chevallier, YY Liu (2014) Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 509:600
Pretzsch H, T Rötzer, R Matyssek, TEE Grams, K-H Häberle, K Pritsch, R Kerner, J-C Munch (2014) Mixed Norway spruce (Picea abies [L.] Karst) and European beech (Fagus sylvatica [L.]) stands under drought: from reaction pattern to mechanism. Trees 28:1305-1321. doi: 10.1007/s00468-014-1035-9
Reth S, M Seyfarth, O Gefke, H Friedrich (2007) Lysimeter Soil Retriever (LSR)—a new technique for retrieving soil from lysimeters for analysis. J Plant Nutr Soil Sci 170:345-346. doi: doi:10.1002/jpln.200625069
Scherer-Lorenzen M, C Körner, E-D Schulze (2005) The Functional Significance of Forest Diversity: A Synthesis. In: Scherer-Lorenzen M, Körner C, Schulze E-D (eds) Forest Diversity and Function: Temperate and Boreal Systems. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 377-389